Gold Atomic Number
Gold Atomic Number Electron Configuration
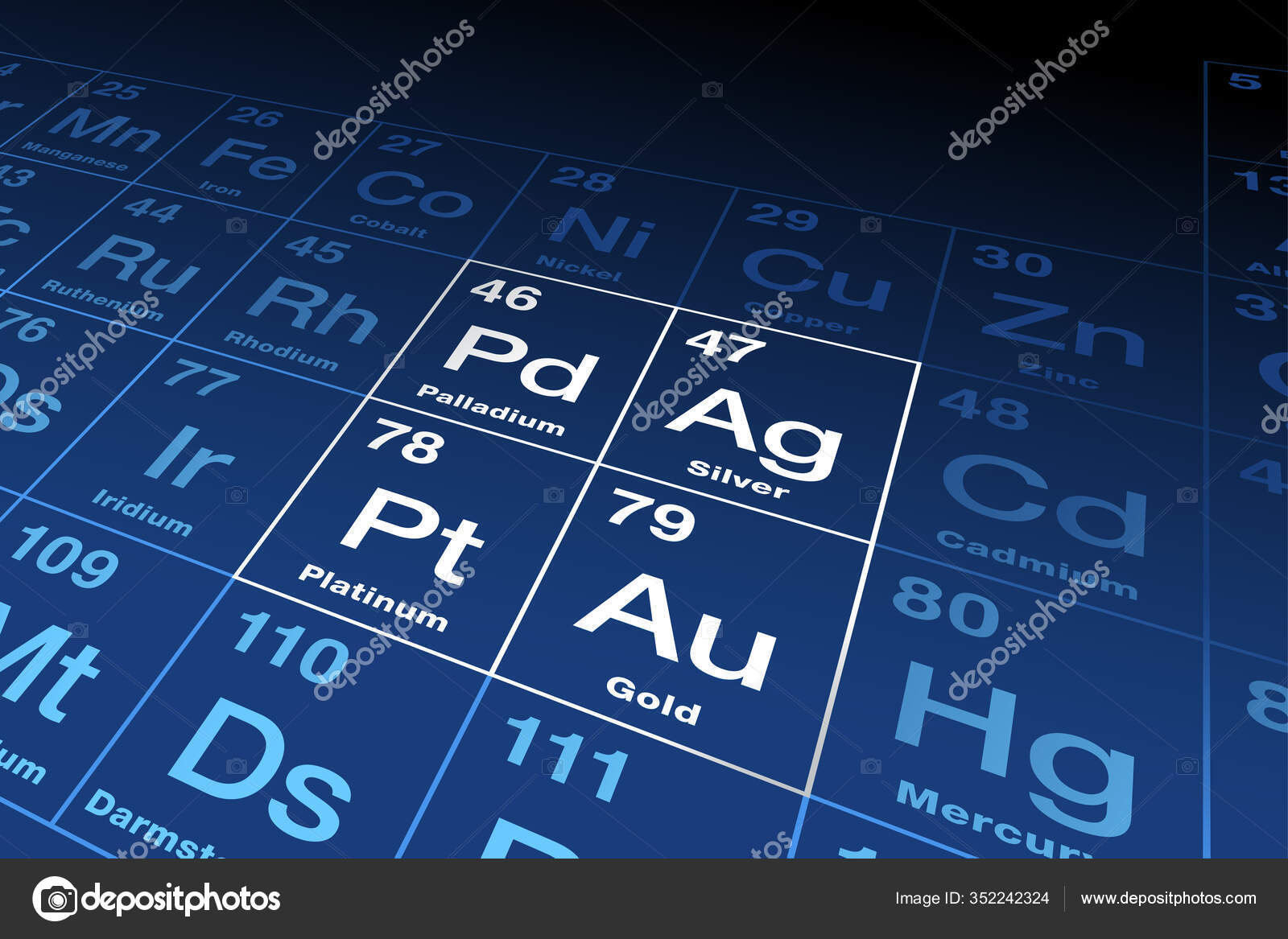
The state of gold in its natural form is solid. Gold is a chemical element with a metallic yellow appearance and belongs to the group of transition metals. The atomic number for gold is 79. The chemical symbol for gold is Au. The melting point of gold is 1337.33 degrees Kelvin or 1065.18 degrees Celsius or degrees Celsius.
Gold: atom sizes. Atomic radius (empirical): 135 pm Molecular single bond covalent radius: 124 (coordination number 2) ppm; van der Waals radius: 245 ppm; More atomc size properties. Atomic Number of Gold Atomic Number of Gold is 79. Chemical symbol for Gold is Au. Number of protons in Gold is 79. Gold Buyers, Smelters, Refiners, Assayers and Brokers of all Gold Metal ATOMIC NUMBER – 79 – ELEMENT SYMBOL – Au ATOMIC WEIGHT – 196.96655 – MELTING POINT – 1064.18 DEGREES C. / 1947.52 DEGREES F. The atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. The atomic number can provide insight into the electronic configuration of the element. For example, carbon has an electron configuration of He 2s 2 2p 2, since its atomic number is 6.
Our editors will review what you’ve submitted and determine whether to revise the article.
Join Britannica's Publishing Partner Program and our community of experts to gain a global audience for your work!
and our community of experts to gain a global audience for your work! Gold Atomic Number Is
Gold (Au), chemical element, a dense lustrous yellow preciousmetal of Group 11 (Ib), Period 6, of the periodic table. Gold has several qualities that have made it exceptionally valuable throughout history. It is attractive in colour and brightness, durable to the point of virtual indestructibility, highly malleable, and usually found in nature in a comparatively pure form. The history of gold is unequaled by that of any other metal because of its perceived value from earliest times.
| atomic number | 79 |
|---|---|
| atomic weight | 196.96657 |
| melting point | 1,063 °C (1,945 °F) |
| boiling point | 2,966 °C (5,371 °F) |
| specific gravity | 19.3 at 20 °C (68 °F) |
| oxidation states | +1, +3 |
| electron configuration | [Xe]4f145d106s1 |
Gold Atomic Number Mass
Gold Symbol Periodic Table
- key people
- related topics
